Connecting chains
Konstanz chemists demonstrate how the chain length of fatty acid molecules can be doubled
Synthetic materials do not need to consist of petroleum: One of the most interesting chemical building blocks used to produce synthetic materials are fatty acids. This production method can be used to create bio-plastics from vegetable oils, e.g. from rape seed or, in the future, from algae. From a chemical perspective, synthetic polymer materials consist of large molecule chains. In order to be able to produce them from vegetable oils, shorter chains of fatty acid molecules are combined piece by piece to form longer chains, which then crystallise into synthetic materials. A team of chemists led by Professor Stefan Mecking from the University of Konstanz have developed a procedure for effectively doubling the length of the effective chain segments. These very long chains improve crystallisation conditions for emerging bio-based plastics and offer advantageous material properties. The research findings were published in May 2017 in the scientific journal “Angewandte Chemie”.
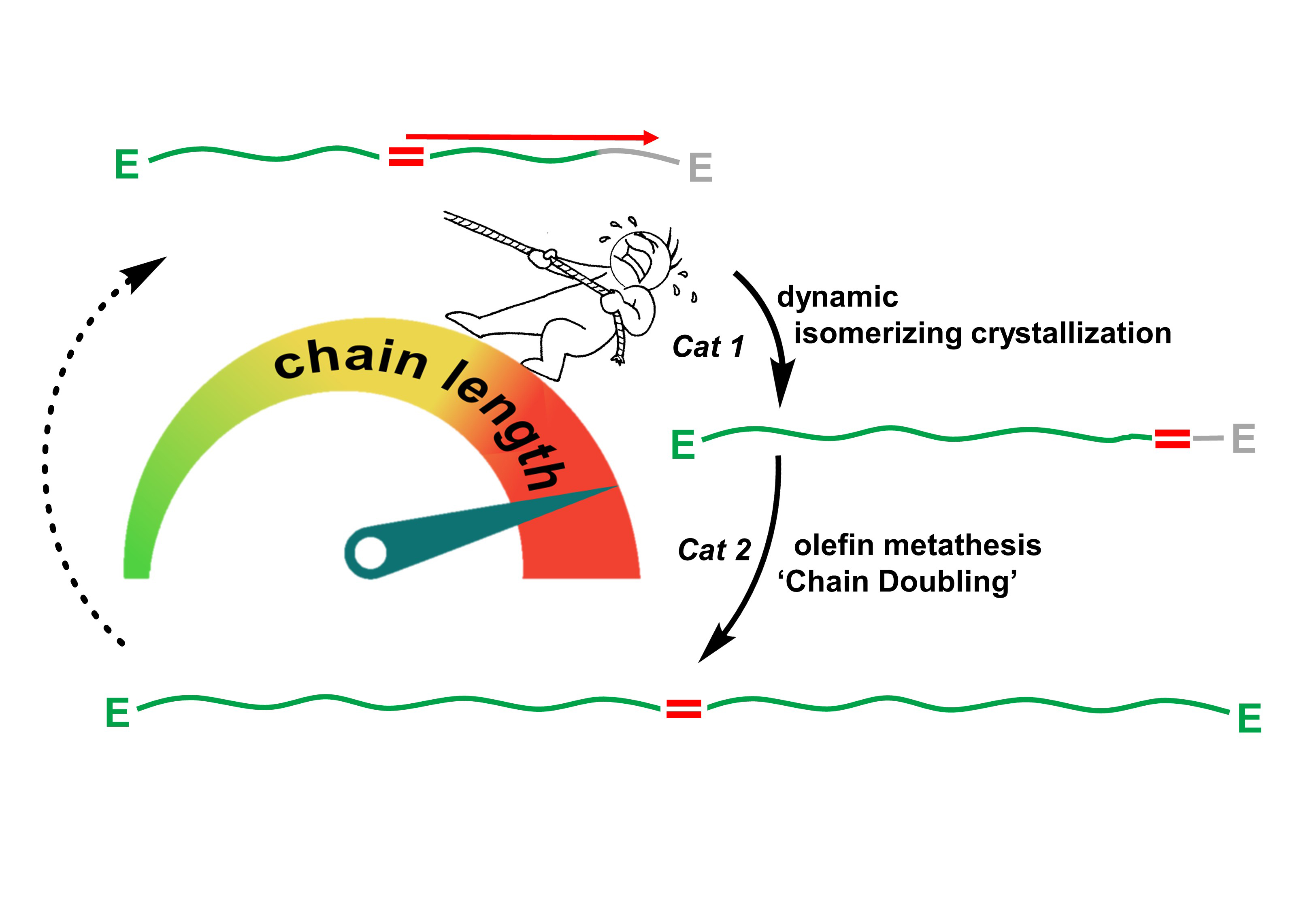
An advantageous feature of a fatty acid in terms of its chemical structure is the occurrence of a reactive group in its molecule chains. This group is the chemical connection point or link through which the individual chains can be connected. The only problem is, however, that this group is typically located in the centre of the molecule. As a result, the chain segments are not connected at the ends, but instead, at a centrally located link. Therefore, effectively half of the possible chain length is not utilised. The perfect solution to this problem would be to relocate the connection point from the centre of the chain to one of its ends. Exactly this is what the chemists in Konstanz accomplished.
“The linchpin of this reaction is the so-called dynamic isomerizing crystallization”, explains Stefan Mecking. First, a catalyst ensures that the functional group begins to move: It shuttles constantly back and forth, from one end of the chain segment to the other. “This dynamic equilibrium creates the condition where the functional group can be fixed at one end of the chain”, describes Manuel Häußler, a doctoral student contributing to this research project. Thus, the chemists can identify the exact moment when the functional group reaches one end of the chain in order to then embed this group at this exact location via a crystallization. The result of this process are molecule chains whose connection points are now located at one of their ends. Now it is possible to connect individual chains at their ends – rather than their centres - through which the lengths of the chain sections are effectively doubled.
This concept is utilized by the Konstanz chemists in a continuously catalyzed process to create optimal building blocks for a longer synthetic chain. Stefan Mecking notes that the resulting synthetic material has improved crystallization characteristics because “the longer chain segments have improved capacities to crystallize”. A special feature of this process is that virtually all of the fatty acid is converted into synthetic material, which leaves almost no leftover material behind: Since the entire chain length is utilised and no portion is “discarded”, there is no waste. Furthermore, large scale co-reagents need not to be involved in these processes. Read more...(only in German)
